LevelBasic
LC is a technique that can be used for the separation of polar and non-polar compounds, for charged and neutral compounds, and for volatile and non-volatile compounds.
HPLC principle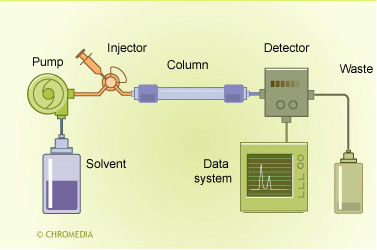
In particular, when mixtures of compounds with different polarities, i.e., drug and their metabolites, parent compounds and their degradation products, should be measured, LC is the method of choice. An overview on the selection of the phase systems can be found in the table:
| Analytes soluble in aqueous solvents | Ionic (acidic, basic, amphoteric) |
|
|
| LC | RP-IS | RP-IP | IE |
| Sorbent | Non-polar | Non-polar | Polar |
| Eluent | Modifier / Buffer | Modifier / Buffer + IP reagent | Buffer |
| Analytes soluble in aqueous solvents | Non-ionic |
|
|
| LC | RP | Partition |
|
| Sorbent | Non-polar | Polar |
|
| Eluent | Modifier / Buffer | Modifier / Buffer |
|
| Analytes soluble in organic solvents | Ionic (acidic basic, amphoteric) |
|
|
| LC | RP-IS | RP-IP | Partition |
| Sorbent | Non-polar | Non-polar | Polar |
| Eluent | Modifier / Buffer | Modifier / Buffer + | Modifier / Buffer |
| Analytes soluble in organic solvents | Non-ionic |
|
|
| LC | RP | Partition (Adsorption) |
|
| Sorbent | Non-Polar | Polar |
|
| Eluent | Modifier / Buffer | Organic solvents |
|
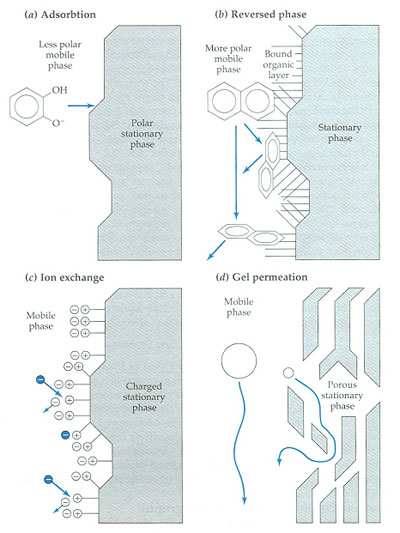
Different mechanisms of retardation in liquid chromatography (J.F. Rubinson, K.A. Rubinson, Contemporary Chemical Analysis, Prentice Hall, Upper Saddle River, 1998).
Adsorption LC
Adsorption LC probably is one of the oldest types of chromatography. It is using a mobile liquid or gaseous phase that is adsorbed onto the surface of a stationary solid phase. The equilibration between the mobile and stationary phase accounts for the separation of different solutes. In this mode a polar sorbent (i.e., silica, alumina) in combination with a non-polar eluent is used. The limited stability and reproducibility, as well as the bad peak shape which is frequently inherent with the analysis of neutral (basic) compounds explains its limited popularity. In most applications adsorption LC can be replaced by normal-phase (NP) partition chromatography.
Nowadays, adsorption chromatography has been replaced by partition chromatography. This form of chromatography is based on a thin film formed on the surface of a solid support by a liquid stationary phase. The solutes equilibrate between the mobile phase and the stationary liquid. Partition chromatography can be performed in the NP and in the reversed-phase (RP) mode.
Normal-phase LC
NP-LC is performed by using silica sorbents which or chemically modified with polar and/or hydrophilic functional groups and in combination with non-polar eluents (Figure 2.67). The most popular sorbents are the amino- and the cyanopropyl sorbent. Both sorbents can be used for the separation of neutral polar compounds. The cyanopropyl sorbent can be used in combination with both polar and non-polar eluents. The use of NP-LC can be advantageous in case of fluorescence detection. Adsorption chromatography (LSC)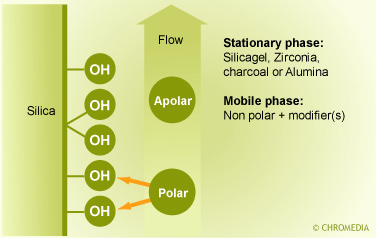
Reversed-phase LC
The most popular mode of LC is RPLC. In this case a non-polar sorbent is combined with polar eluents. The positive features of RPLC are a fast equilibration, excellent stability, good reproducibility and peak symmetry, as well as a large application area. For neutral compounds, in principle, RP-LC is the method of choice. The most popular sorbents are the cyanopropyl, octyl and octadecyl phases. In case a more hydrophobic sorbents or an extreme pH value (<2 or >8) must be applied, polymeric (i.e. copolymers of styrene-divinylbenzene) or graphitized carbon can be applied. RPLC has it own Topic Circles for more detail.
Ion-exchange LC
IEC is performed using either resin (polymeric sorbent) or silica gel material which are chemically modified with charged functionalities (Figure 2.68). These functionalities are covalently attached to the sorbent. Solute ions of the opposite charge in the eluent are attracted to the sorbent by means of electrostatic forces. IEC is applied for the separation of ionized compounds. Depending of the ion-exchanger, weak or strong, the retention is controlled by the ionic strength or the pH, respectively.
Principle of ion-exchange chromatograph 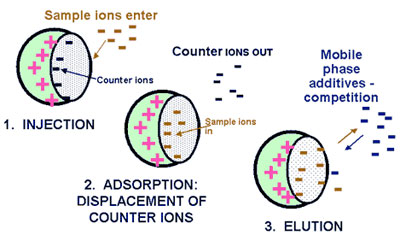 www.forumsci.co.il
www.forumsci.co.il
Ion-pair LC
In IP-LC charged counter-ions are used in combination with a neutral, normally hydrophobic, sorbent. IP-LC can be applied as an alternative for IEC, for the separation of charged compounds. The disadvantage of IP-LC is that, depending on the counter-ion used, equilibration is rather time-consuming.
Ion-suppression LC
IS-LC is a special mode of RP-LC in which the pH of the eluent is adapted in such a way that the ionization of the analytes is suppressed. This approach is mainly used for the separation of weak acids.
 Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class.
Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class. 




