Introduction
Henk Claessens, University of Eindhoven, NetherlandsAbstract Reverse Phase Liquid Chromatography (RPLC) is an extremely important subtechnique of HPLC employing an important subset of the bonded phase chromatography. The technique is easily recognizable since, in comparison to normal or straight phase techniques, it reverses the polarity of the original adsorbent as well as the polarity of the mobile phase.
LevelBasic
Until the nineteen-sixties, the separation of 'non volatile analytes' was often performed by flash, paper, and thin-layer ![]() chromatography. These techniques were:
chromatography. These techniques were:
- Slow,
- Lacked sufficient separation power,
- Did not quantitate reliably.
During the sixties, new theoretical insights accompanied by important developments in column packing technology and chromatographic equipment paved the way for what is now called High Performance or High Pressure Liquid Chromatography (HPLC). The new technique provided:
- Much higher resolution,
- More accurate quantitative results,
- Shorter analysis times in comparison to the earlier techniques.
Since its introduction, ![]() HPLC has evolved into an indispensable tool in many analytical laboratories and is applied to diverse analytical problems.
HPLC has evolved into an indispensable tool in many analytical laboratories and is applied to diverse analytical problems.
Given the advantages and its high separation potential, RPLC has become (with the exception of large molecules) the separation mode of choice for the often simultaneous separation of nonpolar, polar, and ionic analytes. Not surprisingly, therefore, RPLC is used in a large and still growing number of application fields. It has become an indispensable analytical technique in ![]() many areas
many areas
RPLC is the dominant analytical and preparative technique in many other scientific and industrial settings as well. The technique is still developing, in spite of its 40+ years of use, and is a lively subject of academic and industrial research directed towards the development of new columns, instrumentation, and theory.
Development of LC
In the early days of ![]() LC, straight or normal phase chromatography and also liquid – liquid chromatography were the major separation modes available. These techniques required relatively long equilibration times, which seriously hampered efficient method development and sample throughput in the laboratory.
LC, straight or normal phase chromatography and also liquid – liquid chromatography were the major separation modes available. These techniques required relatively long equilibration times, which seriously hampered efficient method development and sample throughput in the laboratory.
Given this difficulty, the discovery of chemically bonded phases (![]() CBP) approximately four decades ago was a historic breakthrough for chromatography. With the advent of CBP’s, different functionalities ranging from non polar to ionic could be covalently bonded or physisorbed onto a substrate. This, in turn, allowed the development of new separation modes, including ion exchange, affinity, and size exclusion chromatography. The following scheme provides an overview of the most important separation modes in LC.
CBP) approximately four decades ago was a historic breakthrough for chromatography. With the advent of CBP’s, different functionalities ranging from non polar to ionic could be covalently bonded or physisorbed onto a substrate. This, in turn, allowed the development of new separation modes, including ion exchange, affinity, and size exclusion chromatography. The following scheme provides an overview of the most important separation modes in LC.
Family tree of separation techniques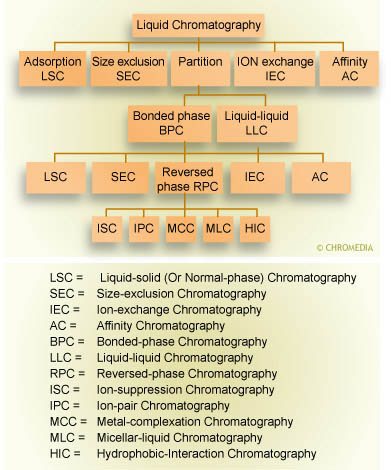
Normal phase versus Reversed Phase
Reverse Phase Liquid Chromatography (RPLC) is an extremely important subtechnique of HPLC employing an important subset of Bonded Phase Chromatography. The technique is easily recognizable since, in comparison to normal or straight phase techniques, it reverses the polarity of the original adsorbent as well as the polarity of the mobile phase compared to the existing ![]() Normal Phase.
Normal Phase.
Its flexibility and applicability to the separation of nearly all types of analytes have contributed to the widespread use and popularity ![]() of RPLC. The following table summarizes a number of the properties of straight and reversed phase chromatography in order to highlight the differences.
of RPLC. The following table summarizes a number of the properties of straight and reversed phase chromatography in order to highlight the differences.
| Comparison of Normal versus Reversed Phase Liquid Chromatography | ||
|
| Normal phase | Reversed phase |
| Stationary phases | Polar; | Moderate to non-polar; |
| Mobile phases | Less polar; non aqueous: | Polar; aqueous: |
| Samples | Insoluble in aqueous solutions | Soluble in aqueous solutions |
| Suitability | Non-ionic organic analytes | All except large analytes |
| Equilibrium time | Lengthy | Short |
As with many other chromatographic techniques, RPLC stationary phases must have sufficient surface area for the different interactions between both the mobile phase and the sample components in order to achieve separation. Furthermore, the sample must be sufficiently soluble in the aqueous-organic mobile phases.
Advantages of Reversed methods
Compared to 'Normal Phase' separation modes, Reversed methods offers a number of distinct advantages including:1. Given the variety of available stationary phase ligands, RPLC can use hydrophobic, polar, ionic, and other interactions to optimize separations. This allows the simultaneous separation of non-polar, polar, or ionic components. In fact, ![]() RPLC can separate nearly all molecules with the exception of large molecules. Conventional hydrophobic, ion-pair (IPC), ion suppression(ISC) and hydrophobic interaction (HIC) chromatography are well-known examples of RPLC separation modes. These separation modes will be discussed in detail in the separate circles around the topic “Separation modes“.
RPLC can separate nearly all molecules with the exception of large molecules. Conventional hydrophobic, ion-pair (IPC), ion suppression(ISC) and hydrophobic interaction (HIC) chromatography are well-known examples of RPLC separation modes. These separation modes will be discussed in detail in the separate circles around the topic “Separation modes“.
2. A large number of high quality and efficient RPLC stationary phases are ![]() available. Combined with the wide spectrum of potential mobile phase mixtures, this offers a range of selectivity adequate to solve nearly any separation problem.
available. Combined with the wide spectrum of potential mobile phase mixtures, this offers a range of selectivity adequate to solve nearly any separation problem.
3. Water frequently forms an inexpensive, non-toxic and major part of the mobile phase. In addition, many samples to be analyzed are soluble in aqueous-organic mixtures.
4. Standard RPLC stationary phases offer acceptable chemical and thermal stability. These stationary phases can typically tolerate mobile phase pH’s from 2.5 to 7.5 and column temperatures up to 50º C, which is sufficient for a great number of separations. Recently, other RPLC phases have become available which can tolerate pH’s in the range of 2 to 12 and can handle temperatures up to 90º C and beyond. These recent developments have expanded the working range of RPLC substantially.
5. Although RPLC is mostly applied as an analytical technique, it is also used in laboratory and industrial scale preparative HPLC and for the determination of physical-chemical constants.
6. RPLC allows both isocratic and gradient separations with modest equilibration times.
7. Retention and selectivity mechanisms are readily understood and a number of retention models have been developed to support the optimisation of separations. In addition, a number of software programs have become available to support the analyst in fast and automated method development.
Given these advantages and its high separation potential, RPLC has become (with the exception of large molecules) the separation mode of choice for the often simultaneous separation of nonpolar, polar, and ionic analytes. Not surprisingly, therefore, RPLC is used in a large and still growing number of application fields. It has become an indispensable analytical technique in polymer chemistry, biochemistry, food and pharmaceutical chemistry, life sciences and clinical and forensic chemistry, just to mention a few. RPLC is the dominant analytical and preparative technique in many other scientific and industrial settings as well. The technique is still developing, in spite of its 40+ years of use, and is a lively subject of academic and industrial research directed towards the development of new columns, instrumentation, and theory.
Stationary phases & Substrates
Stationary PhasesNonpolar modified stationary phases are by far the most important column packings in RPLC. In the majority of such phases, alkanes or other non polar functional ligands are ![]() covalently bonded to a support material or substrate. The length of the carbon chains bonded to a substrate is typically somewhere between C1 and C22. Among these stationary phases, octyl (C-8) and octadecyl (C-18) modified silicas are well-known and are often used as standard column packings.
covalently bonded to a support material or substrate. The length of the carbon chains bonded to a substrate is typically somewhere between C1 and C22. Among these stationary phases, octyl (C-8) and octadecyl (C-18) modified silicas are well-known and are often used as standard column packings.
Columns and stationary phases for RPLC must provide:
- Retention and selectivity sufficient to solve a specific analytical problem.
- High efficiency in order to achieve acceptable resolution
- Satisfactory column longevity resulting from adequate chemical, mechanical and thermal stability.
- Excellent batch to batch and column to column reproducibility and long term availability.
- Acceptable pressure drop
Substrate types
RPLC stationary phases can be manufactured from different susbtrate types, for example: inorganic oxides such as silica, alumina, zirconia; or organic polymers like styrene-divinylbenzene, methacrylates and graphitized carbons. ![]() In general, substrates for RPLC phases should meet the following major requirements:
In general, substrates for RPLC phases should meet the following major requirements:
- High mechanical strength adequate to withstand pressures of 500 bar and higher.
- Uniformly shaped pores with a narrow size distribution (Ängstrom range) and sufficient porosity, usually expressed as ml/gr.
- Large and homogeneous surface area; usually expressed as m2/g or m2/ml.
- Uniformly shaped particles (μm range) with a narrow particle size distribution.
- No swelling or shrinking upon exposure to solvents.
- Chemical and thermal stability under different experimental conditions.
- High purity; free of metals contamination.
Silica meets nearly all of these requirements and, in fact, is a dream substrate for the synthesis of RPLC phases. Silica falls short only in terms of chemical stability since it begins to dissolve at a pH of approximately 7.5 . New synthesis and modification techniques, however, have resulted in greatly improved silica substrates and stationary phases thereof showing ![]() high chemical and thermal stability.
high chemical and thermal stability.
Silica substrates
Silica substrates can be manufactured by either silgel or solgel procedures. The choice influences the final substrate and stationary phase’s surface area as well as its chemical stability. The chromatographic properties of the phase are determined by the chemical structure of the surface and by its Specific Surface Area (SSA), which is inversely proportional to the pore diameter of the particles. Hence:
- The smaller the pores, the more active surface area will be available for the chromatographic separation process.
The specific surface area and the pore and particle diameter can be adjusted by changing the manufacturing synthesis conditions. Silica substrates are extremely porous, as evidenced by their high specific surface areas.
The illustration hereunder shows the schematic structure of a silica ![]() substrate. In this figure the different structural elements present on a silica surface are presented:
substrate. In this figure the different structural elements present on a silica surface are presented:
- The silanol groups become the anchoring groups for the subsequent synthesis of covalently bonded RPLC phases.
- The reactivities of the isolated, vicinal and geminal silanol groups are substantially different, while siloxane groups have hydrophobic properties.
- The figure also illustrates a situation in which a metal impurity withdraws electrons from a neighboring silanol, making the proton more acidic.
Active groups at surfaces of silica substrates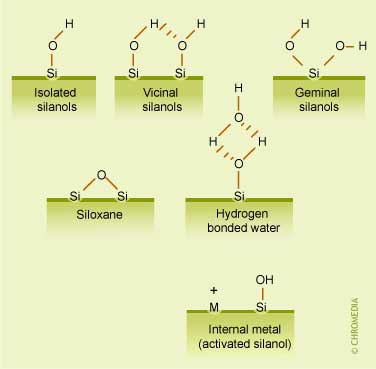
Silica can be produced with many different morphologies, porosities and surface areas. For example, silica can be manufactured:
- In well defined irregularly or spherically shaped particles of specific sizes and of controlled porosities and pore diameters.
- In addition to these more
 common morphologies, silica is also widely used for the manufacture of monolithic, non- porous and pellicular RPLC stationary phases.
common morphologies, silica is also widely used for the manufacture of monolithic, non- porous and pellicular RPLC stationary phases.
Below are some typical ranges for the various properties of the silica substrates used for HPLC:
Structures of different silica substrates
Silica substrates
Specific surface: 5 to 1000 m2/g
- Particle sizes : 1.5 to 10 micrometer
- Pore size: 30 to 4000 Angström
- Pore volume: 0.8 to 2.0 ml/g
- Number of silanol groups: ~8 micromol / nm2
Not surprisingly, therefore, the majority of HPLC stationary phases are produced from silica substrates and a wide variety of phases bonded with various non- polar, polar and ionic functional groups are marketed. Among these CBP’s, stationary phases for RPLC are an important group of packing materials.





